How to Package and Ship Category B (UN 3373) Biological Substances
Category B biological substances are a dangerous good, specifically pathogens that present a non-fatal risk in the event of a release and therefore are not considered Category A. If you need a refresher on which category your substance falls under, see our companion article. Here, we’ll review requirements for packaging, labeling, marking and documentation.
UN 3373 Packaging Instructions
Follow and save any instructions provided with boxes and validated thermal shippers you purchase, so you always have something to reference. Triple packaging is the best practice for all classifications of biological shipments.
Here are the four elements you need for your packaging:
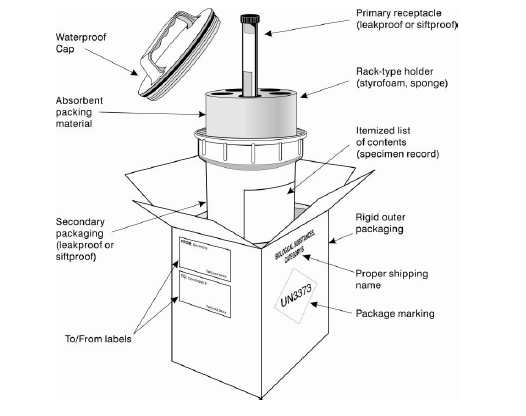
1. Leak-proof Primary Receptacle
- This contains your sample — make sure it has a leak-proof seal, tape or parafilm screw cap.
2. Leak-proof Secondary Packaging
This is your inner container. Ensure the primary or secondary receptacle can withstand:
- Pressure: not less than 95kPa.
- Temperature:- 40°C to 55°C
3. Cushioning and Absorbent Material
- If you have a liquid substance, sufficient absorbent material must be included to absorb the entire contents of all primary receptacles. Acceptable absorbent materials include cellulose wadding, cotton balls, super-absorbent packets, and paper towels. This generally goes in between the primary and secondary packaging.
- Cushioning material is also necessary — and needs to be distinct from absorbent material. Generally this is between the secondary and outer packaging.
4. Rigid Outer Packaging
- The outer box must be able to meet a drop test of 1.2m.
- The smallest external dimension must be at least 100x100mm — look to the manufacturer’s instructions for this info.
- Before sealing the outer packaging, you must make an itemized list of the contents of the package and enclose the list between the secondary packaging and outer packaging.
Note: This does not include Styrofoam layer, if using wet/dry ice. Express carriers such as FedEx and UPS do not accept packages with a Styrofoam container as the outside packaging so add rigid outer packaging such as a cardboard box or validated thermal shipper.
Maximum Permissible Quantities
Driving courier, FedEx Ground and UPS Ground services: No restrictions
Air including FedEx Express, UPS Air and Specialty Cold Chain:
| Liquid | Solid | |
| Per primary receptacle (sample) | 1L | 4kg |
| Per outer container | 4L or 4x1L bottles | 4kg |
Example of the triple packaging system for the packing and labeling of Category B, UN 3373 infectious substances:
Disposing of or Repurposing Rigid Outer Packaging
Before empty packaging is returned to the consignor, or sent elsewhere, it must be disinfected or sterilized to nullify any hazard and any label or mark indicating that it contained an infectious substance must be removed.
When shipping blood samples
A biohazard symbol is required on any sample that contains human blood and other potentially infectious materials (OPIM). This label must be affixed to the primary receptacle or secondary packaging.
Overpack
An overpack is an extra outside packaging used when shipping multiple boxes to the same address or used to refrigerate materials being shipped in a smaller box. All markings and labels must be on the outside of the box along with the word “OVERPACK”. Since your inside package is the “triple package” you need all markings and labels on that package as well.
UN 3373 Labeling Instructions
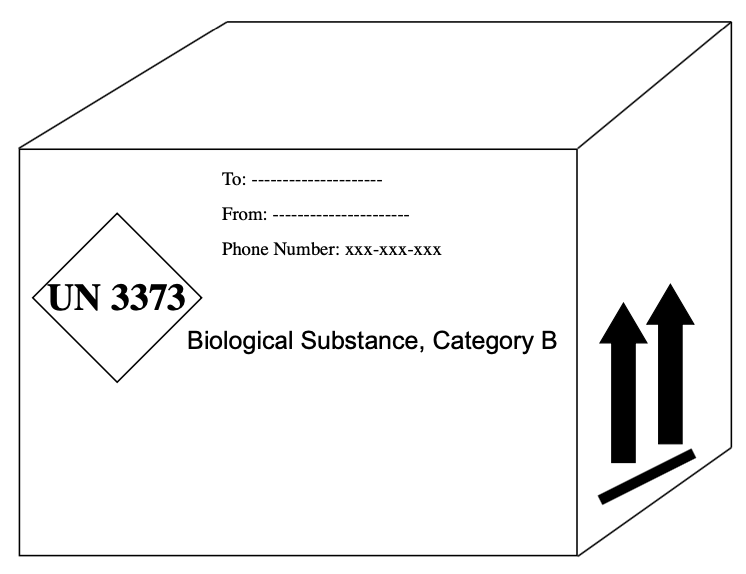
Standardized labeling must be affixed to the outside. When the package dimensions are adequate, labels must be located on the same surface of the package near the Proper Shipping Name mark.
Except for the orientation arrows, mount all labels and markings on the front side of the box — all on one surface area. “Cargo Aircraft Only” labels are required when applicable.
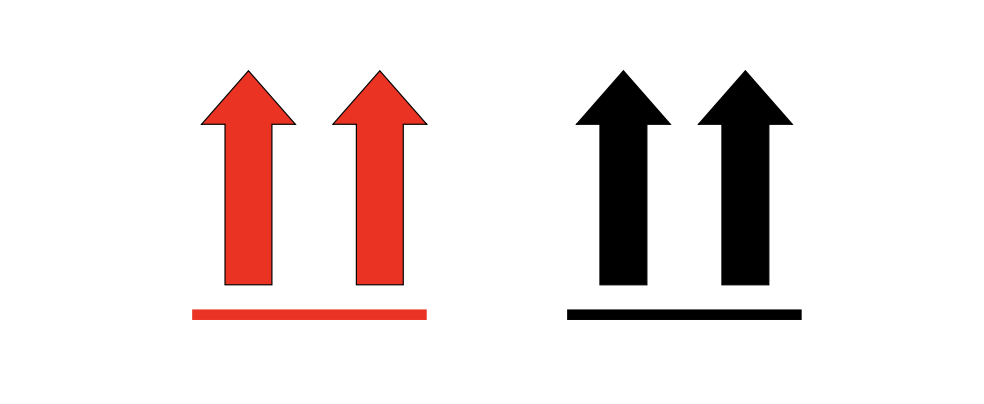
Orientation Arrows Label
If you are shipping a liquid, orientation arrows are required. Label must be affixed on 2 opposite sides and perpendicular to the front of the package. Orientation arrows should be red or black on a contrasting background.
Overpack Label
Overpack labels have a height Requirement of 12mm (~0.5 inch). Either of these styles is acceptable:

Marking
The markings on a package are the words and numbers that must be present. Generally, markings include:
- Proper Shipping Name
- UN # Net Quantity (Category A Infectious Substance and Dry Ice only)
- Contact Information
- Emergency Contact Number: 24 hour, uninterrupted, phone number where someone can be reached about the package while it is in transport
- Responsible Person: Name and number must be listed on outside of package
- Under IATA regulations, Category B, Biological Substance and GMMO Diamonds are each considered markings as well.

- Diamond must be 2” x 2” or 50mm X 50mm
- All letters and numbers must be 6mm tall
- Outside border of the diamond must be 2mm thick
Tip: Don’t include any abbreviations other than mL, L, G, KG.
UN 3373 Documentation Requirements
All Shipments must include:
- Bill of Lading (if ground transport), or Airway Bill (if air transport)
- “UN 3373 Category B Biological Substance x 1 pkg”
- Appearance of form will vary between carriers
- 5 copies of the Commercial Invoice for international shipments
- Include an itemized list of contents between secondary and outer packaging (not inside secondary)
- It's best practice to save all commercial Invoices, airway bills and lists of contents in a binder for at least two years.
Contact the Receivers
Make sure you contact the receivers prior to shipping. Keep in mind that some labs only receive Monday to Thursday. If you ship on Friday, it might sit over the weekend, or arrive on Saturday and nobody's there. If they are open on Saturday, make sure you label for Saturday delivery so the carrier knows they can deliver on Saturday and not hold the package over the weekend.
Know the stability of your sample and how long it can last, roughly 6% of Express Carrier shipments are not delivered on the requested date for a variety of reasons. Think carefully of the day you’re shipping and the stability of the shipment to avoid a potential re-draw from the patient.
Other FedEx and UPS Requirements
- DO NOT put your package in a FedEx or UPS drop box
- Use a FedEx or UPS pouch and insert the airway bill so that it is laying flat-not folded.
- Be sure to remove the sender’s copy and save this documentation for 2 years. Affix the adhesive pouch to the top of the fiberboard box.
- If there is a commercial invoice (5 copies is good practice) to include for international shipments, you may fold these together in half and place them behind the airway bill inside the pouch.
- When scheduling a FedEx or UPS pickup, make sure to explain you're shipping UN3373B. Only certain drivers are certified to collect hazardous materials.
International shipments
Before you ship any material abroad, you should answer the following questions:
- What are you shipping and is an export or import license required?
- Who will receive it (are they on the Restricted Parties List)?
- Where is it going (is it on the Embargoed Destinations list)?
- What are they going to do with it?
- Is the shipment over $2,500 or subject to a license? If so, you need to file a report with the U.S. Census called an Electronic Export Information (EEI).
- International shipments require a commercial invoice.
Penalties and Fines
Shipping biological substances is a tightly regulated activity. If you need more reasons to get it right, here is a list of violations and the minimum assessments for each:
- No UN #: $1,000-2,000
- No Shipping Name: $1,000
- No Emergency #: $2,600
- Violations related to Select Agents 42 CFR 73: $250,000/individual; $500,000 facility
Need more assistance? Consider working with a company like Mercury Business Services. Mercury offers a comprehensive shipping solution that allows you to focus on your work, and leave the shipping and logistics to the professionals.
Related Content
How To Classify Biological Substances For Shipment
Shipping Exempt Human & Animal Specimen
UN 2814 and UN 2900 Infectious Substances Shipping
Import Assistance for Animal Specimens into the U.S.
Hazardous Materials
At Mercury, we prioritize safe and compliant transportation of goods. To assist our clients, we've created a guide on shipping Dangerous Goods (DG).
Differences Between UN 2814, UN 2900 and UN 3373
August 3, 2023Shipping hazardous materials required understanding various classifications and regulations to ensure maximum safety. In biological substance shipping, the commonly referenced categories are UN 2814/UN 2900 (Category A) and UN 3373 (Category B).

